Cost & Quality Problems
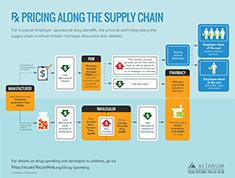
Prescription Drug Costs
Prescription drug costs currently account for 15 percent of overall health spending and are once again coming under scrutiny as important drivers of health care spending.1
Spurred by blockbuster drugs like Lipitor and Plavix going off patent and increased use of generics, drug spending has moderated in the last decade since the high growth period of over decade ago.2 Three important trends are bringing this period of low drug spending growth to an end: increased spending on prescription drugs, the diminishing cost-saving impact of generic drugs and price gouging by drug manufacturers.
Brand Name Drugs
Retail prices for brand name prescription drugs increased by an average of 5.8 percent in 20183—more than double the inflation rate. Studies have also shown that price increases, not innovation, are driving prescription drug cost growth. A report published in Health Affairs found that more than 85 percent of the annual increases in the average weighted cost of brand-name drugs between 2008 and 2016 was attributable to existing drugs and less than 15 percent to new drug products.4 Additionally, pharmaceutical and biotechnology sales revenue increased from $534 billion to $775 billion between the same period, while the largest 25 companies maintained profit margins between 15 and 20 percent (more than double that of non-drug Fortune 500 companies).5
High Cost of Specialty Drugs
A form of brand name drugs, the term "specialty drug" does not have a uniform definition. The term broadly comprises drugs that are: high priced; often (but not always) made of biological matter; treat complex conditions; require special handling; or require infusion or injection in a doctor’s office.
The cost of new speciaty drugs is truly prohibitive. In 2016, almost half of the drugs studied by America's Health Insurance Plans cost in excess of $100,000.6 Specialty drugs are used by fewer than 2 percent of privately insured patients in the United States but account for more than 46 percent of all drug spending.7
Generics Becoming More Expensive
Evidence suggests that two generic competitors for a drug can lower the average generic price to nearly half that of the brand name product. Event more generic competitors can lower the average generic price to 20 percent of the price of the brand name product.8
Unfortunately, high rates of generic substitution will continue but will do less and less to bring down overall spending. In coming years, fewer expensive brand name drugs are expected to go off patent.9
A second reason generics are less likely to moderate future spending is that the costs of many generic drugs are increasing. The reason for these generic costs increases are complex and are due to many factors, from raw material shortages to generic competitors leaving the market. But whatever the reason, many drugs that have been historically inexpensive have seen sharp price increases. HCCI found that the average annual price growth for generic medications was 4 percent in 2016.10 However, some generic drug manufacturers, like Teva, Mylan and Novartis, have come under fire for inflating the prices of generic drugs as much as 1000 percent.11
Biosimilars
As more drugs take the form of biologics, the impact of generics is reduced. Biologics are made of biological matter and for that reason, cannot be replicated exactly. Instead, biosimilars can be introduced into the market. Biologics have a 12-year period of market "exclusivity," meaning that the U.S. Food & Drug Administration (FDA) cannot approve a biosimilar during this period.
Impact on Consumers
These cost trends mean higher premiums for consumers, higher cost-sharing and sometimes even illegal, discriminatory formulary designs to deter enrollees who need expensive medications. Another market response to high costs is to limit patient choice. For example, the nation’s largest pharmacy benefits manager, CVS, negotiated an exclusive deal with and AbbVie Inc., a drug maker of an alternative to Sovaldi, in exchange for a discounted price.
Options for Curtailing Rx Costs
Options for curtailing high Rx costs include:
Federal
- Reforming the way monopolies are granted to drug companies to incentivize innovation
- Greater oversight over pricing
- Increased use of comparative effectiveness research to help identify the relative value of medications
State
- Generic and biosimilar friendly state substitution laws
- Prescription drug oversight entity
- Requiring price transparency from manufacturers
- Anti-price gouging/price setting
- Reference pricing
- Allowing importation from Canada
- Volume purchasing/subscription
- Regulating Pharmacy Benefit Managers (PBMs)
It will take a sustained effort by consumers, payers, purchasers working at both the state and federal level to make headway on these issues and ensure that health care costs are to be sustainable and access to life saving drugs is preserved. In the short-run, advocates may want to consider getting involved in benefit design rules being discussed in state Marketplaces to try to mitigate the impact of high drug costs on sicker enrollees.
Notes
1. Yu, Nancy L., Preston Atteberry and Peter B. Bach, “Spending On Prescription Drugs in the US: Where Does All the Money Go?” Health Affairs Blog (July 31, 2018)
2. Martin, Anne B, et al., “National Health Spending in 2012: Rate of Health Spending Growth Remained Low for the Fourth Consecutive Year,” Health Affairs, Vol. 22, No. 1 (January 2013).
3. Purvis, Leigh, and Stephen Schondelmeyer, Rx Price Watch Report: Brand Name Drug Prices Increase More than Twice as Fast as Inflation in 2018, AARP Public Policy Institute (November 2019).
4. Hernandez, Immaculada, et al., The Contribution of New Product Entry Versus Existing Product Inflation in the Rising Costs of Drugs, Health Affairs, Vol. 38, No. 1 (January 2019).
5. United States Government Accountability Office, Drug Industry: Profits, Research and Development Spending, and Merger and Acquisition Deals (November 2017).
6. Light, Donald W. and Kantarjian, Hagop, Market Spiral Pricing of Cancer Drugs, Cancer (November 15, 2014).
7. IQVIA, Medicine Use and Spending in the U.S.: A Review of 2017 and Outlook to 2022, Durham, N.C. (April 2018).
8. Federal Drug Administration, Center for Drug Evaluation and Research, Generic Competition and Drug Prices (November 20, 2017).
9. Purvis, Leigh, A Sense of Deja vu: The Debate Surrounding State Biosimilar Substitution Laws, AARP (September 2013).
10. Health Care Cost Institute, 2016 Health Care Cost and Utilization Report, Washington D.C. (2018).
11. Murphy, Heather, Teva and Other Generic Drugmakers Inflated Prices Up to 1,000%, State Prosecutors Say, New York Times (May 11, 2019).
- Consumer Reports: EpiPen Costs Add to High-Deductible Insurance Woes (September 12, 2016).
- Consumer Reports: Secret Drug Prices and Their Impact on Consumers (April 12, 2016).
- Wall Street Journal: Are Out-of-Pocket Costs Too High? Lynn Quincy Says "Yes!" (April 10, 2016).
- Wall Street Journal: How Pfizer Set the Cost of Its New Drug at $9,850 a month (December 9, 2015).
- Lynn Quincy testimony before the Democratic Steering and Policy Committee, U.S. House of Representatives (December 2, 2015).
- Hub Research Brief: Primer on Prescription Drug Costs (April 2015).
- New England Journal of Medicine: Measuring the Value of Prescription Drugs (December 31, 2015).
- Wall Street Journal: Why Higher Drug Costs Are Consumers' Biggest Cost Worry (September 8, 2015).
- New York Times: The Solution to Drug Prices (September 9, 2015).
- Washington Post: Cancer Drugs Aren't Just Really Expensive; They're a Bad Value (August 27, 2015).